Which Best Describes Rutherford's Model of the Atom Answers Com
There are no electrons in the Rutherford Model. Which statement best describes rutherfords model of the atom.
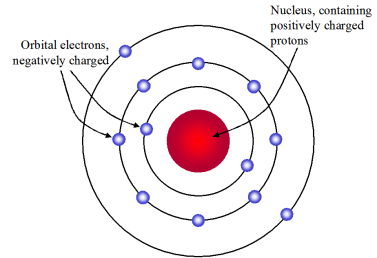
Rutherford Model Which Best Describes Rutherford S Model Chemistry
Which of the following statements BEST describes the location of electrons in Rutherfords model of the atom.

. Rutherfords Model of the Atom Rutherford proposed that each atom has a dense central core which he called the nucleus The nucleus is a central region that is very small relative to the total size of an atom The nucleus contains virtually all of the mass and all the positive charge of the atom Problem- the electron orbits as some distance. According to Rutherford model of the atom. 588 students attemted this question.
Electrons are both inside and outside of the nucleus. O A tiny hard solid sphere that cannot be divided into smaller pieces. Which statement best describes the nucleus of.
2 Major space in an atom is empty. Which best describes the current model of the atom. The modern-day quantum model of the atom is better than john daltons model because it.
The electrons are outside the nucleus. Which statement describes one feature of Rutherford model of the atom. Huge stadium with a positively charged marble at the center.
3 Atoms nucleus is surrounded by negatively charged particles called electrons. B - it is like a aquarium with swimming fish representing positive charges. Rutherfords model of the atom is also known as the planetary model because this is when he discovered the nucleus of the atom.
1 Atoms have their charge concentrated in a very small nucleus. Which best describes Rutherford and model of the atom. It is like a huge stadium with a positively charged marble at the center best describes Rutherfords model of the atom.
Emagnitude of the electrical force acting between a 24 108 c charge and a 18 106 c charge that are separated by 0008 m is n rounded to the tenths place. Rutherfords Model Experiments performed Let us first learn something about the experiments he performed. Three vectors Ā B and C are represented both in magnitude and direction by the three sides of a triangle taken.
It is like a huge stadium with a positively charged marble at the center. Which statement best describes how the cash flow statement differs from the income statement. See answer 1 Best Answer.
The Rutherford model of the atom is basically a planetary model. It is described as follows 1 Atom consists of positively charged small dense nucleus having all the protons and neutrons in it. Rutherfords Model 150818 Rutherford was always curious in knowing about the arrangement of electrons in an atom.
A particle that contains a tiny and positively charged nucleus surrounded by a cloud of electrons. 4 An atom is electrically neutral. D - it is like a huge stadium with positively charged marble at the center.
Which of the following best describes Rutherfords model of the atom. Next What is the simile used to describe the death of the newspapers. Rutherford discovered the proton and supposed the existence of neutron facts that led to an.
Also surrounding the nucleus is an electron cloud. Expert answered emdjay23 Points 161188 Log in for more information. 2 Nucleus is surrounded by electron having negative.
Rutherfords atomic model was based on alpha particle scattering experiment. By performing an experiment using alpha particles and gold foil he came to some conclusions. Suatu atom dengan nomor atom 53 dan massa atom 127 mengandung.
The electrons are inside the nucleus. De revolve in circular path and. It places the bulk of the mass in the center and this nucleus is positively charged and.
Which best describes rutherfords model of the atom. An atomic model of Rutherford doesnt exist. A particle that contains a small and positively charged nucleus with electrons moving around the nucleus.
C - it is like a fried egg with the yolk representing the nucleus. The entire mass of atom is concentrated in nucleus. A - it is like an avocado with the pit representing the nucleus.
He also discovered that the nucleus of the atom located at the center of an atom is only 13000th the size of the atoms diameter.

Describe Rutherford S Atomic Model

Atom Rutherford S Nuclear Model Britannica

Electrons In Atoms Flaws In Rutherford S Atomic Model Discovered Dense Positive Piece At The Center Of The Atom Nucleus Atom Is Mostly Empty Space Ppt Download
0 Response to "Which Best Describes Rutherford's Model of the Atom Answers Com"
Post a Comment